135 years of service
College with Potential for Excellence (UGC)
NAAC Mentor College (UGC)
RUSA (UGC) Funded Institution
3.5 Stars for Innovation from IIC, MHRD, New Delhi
DBT (STAR COLLEGE) Funded
SES REC Institution, MHRD
NIRF INDIA Ranking: 53
Veritas Vos Liberabit (The Truth will set you Free)
Transforming the Youth through Holistic Education towards an Enlightened Society.
- To Ensure Inclusion and Access of Quality Education.
- To Provide an Environment of Learning that enhances Dissemination of Knowledge.
- To Nurture Research and Innovation for the betterment of Life and Progress of the Nation.
- To Undertake Collaborative Partnerships for Facilitating Exposure and Sharing.
- To Impart Social and Environmental Sensitivity in Students through Extension and Outreach.
- To Equip Students with Life Skills in Facing Challenges and Responsibilities.
- To Help Students attain Moral, Spiritual and Emotional integrity.
- Faith in God
- Pursuit of Excellence
- Integrity
- Diversity
- Compassion

OUR PLACEMENT PARTNERS

OUR UPCOMING EVENTS

ACADEMICS

OUR PLACEMENT PARTNERS

SUPPORT AND ASSISTANCE







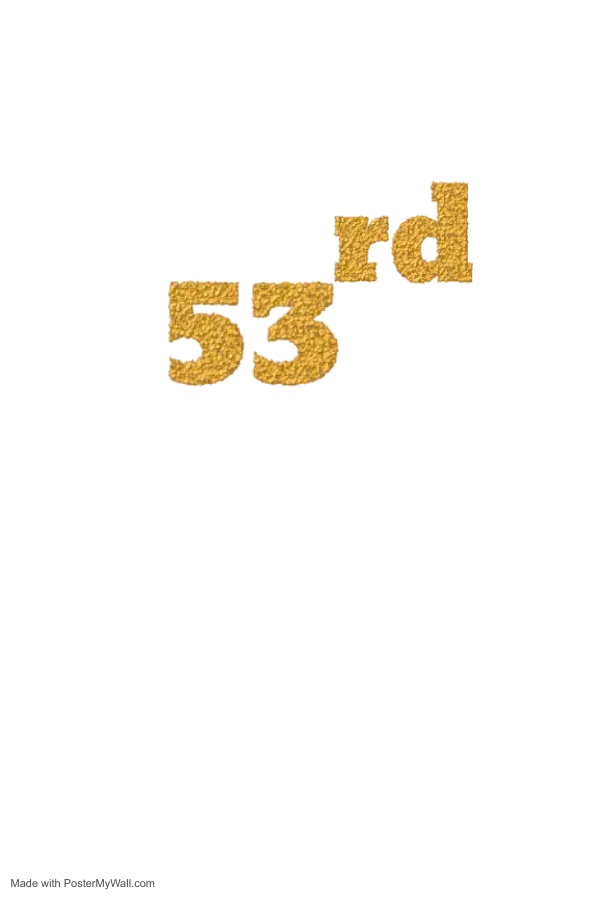


.png)